System
SAGITTA
PRODUCT NAME: MEDIIMPLANTES INTERVERTEBRAL CASE SYSTEM AND ASSOCIATED INSTRUMENTS
COMMERCIAL NAME: SAGITTA SYSTEM
REFERENCE: 111
MANUFACTURER: Mediimplantes S.A.
MANUFACTURING MATERIAL:
PEEK is a material according to ASTM F2026 – Standard Specification for Poly-Ether-Ether-Ketone (PEEK) – Polymer for surgical applications. PEEK offers several advantages over materials traditionally used to develop medical devices. Amongst them, the material is radiolucent, which allows the surgeon better evaluate arthrodesis progress without any type of image.
FUNCTIONAL DESCRIPTION
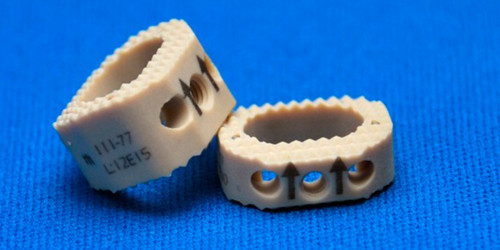
SAGITTA is a system composed of PEEK intersomatic cervical cases offered in different heights. The system is indicated for patients with cervical and degenerative disc pathologies and applied through anterior-approach cervical discectomies and intersomatic arthrodesis. Placement of the PEEK cases is done with the purpose of avoiding bone graft displacement; maintaining disc and foraminal height; restoring yellow ligament folding; avoiding pseudarthrosis; and maintaining cervical spine lordosis.
INDICATIONS
– Cervical pseudarthrosis
– Cervical disc hernia
– Recurrent cervical disc hernias
– Cervical degenerative disc disease
– Cervical instabilities and degenerative discopathies
– Pseudarthrosis and unsuccessful arthrodesis
STUDIES AND TESTS
– Static compression test based on ASTM F2077 – Tests Methods for Intervertebral Body Fusion Devices
CERTIFICATES
– ISO 9001 and ISO 13485 certificates
– INVIMA Sanitary Registration Nr. 2010DM-0006328
PRESENTATION AND PACKAGING
Implants from the SAGITTA System are provided unsterilized, individually packaged, and individually laser-marked. Each laser marking shows: product code, lot number, MEDIIMPLANTES logo, material symbol, and use-specific dimensions. Laser markings are permanent and allow for product traceability, even after implantation.
Primary Packaging: medical-grade paper bag for products to be sterilized and a laminated polyester film with a security band. It’s provided with non-toxic chemical indicators, fit for commercial sterilization methods, allowing for internal monitoring of sterilization parameters.
Secondary Packaging: Organizing racks that protect the implant from any possible mechanical damage caused by product movement to other locations.
Tertiary Packaging: Aluminum cases with safety mechanisms that contain all available organizing racks to display in an organized fashion for the surgeon’s use.
STORAGE AND AVAILABILITY
Immediate availability and easy storage. Does not need refrigeration nor any special handling procedures asides from maintaining cleanliness conditions to guarantee sterilization.