System
INFINITY
PRODUCT NAME: POSTERIOR CERVICAL AND OCCIPITAL SYSTEM
COMMERCIAL NAME: INFINITY
REFERENCE: 107
MANUFACTURER: Mediimplantes S.A.
MANUFACTURING MATERIAL: Ti 6Al 4V ELI (Extra low interstitial) Alloy for Surgical Implant Applications – UNS R56401, ASTM F136-02.
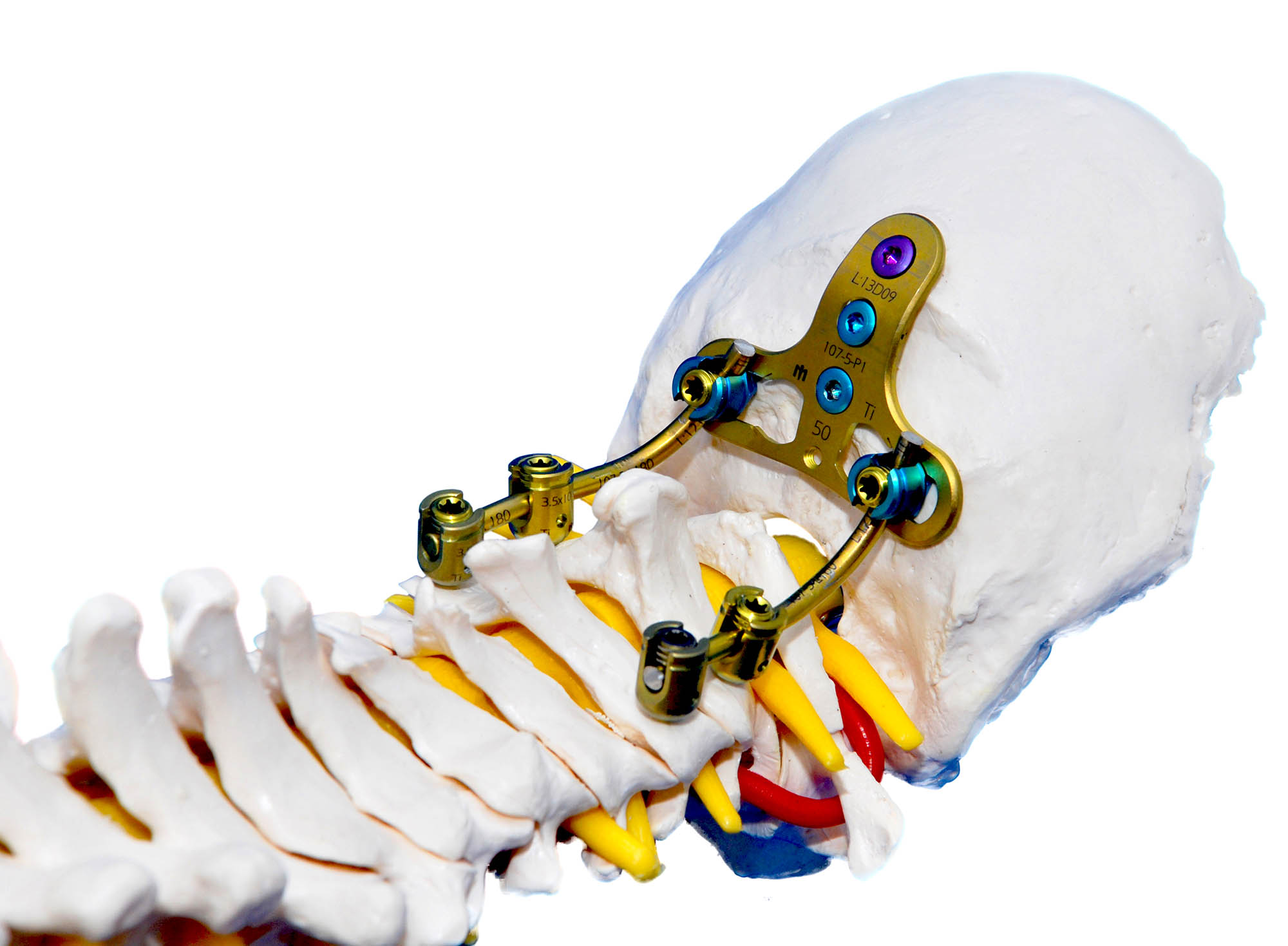
FUNCTIONAL DESCRIPTION
INFINITY is a system designed to perform fixation, immobilization, and stabilization of cervical spine segments.
The system has two elements for occipitocervical joint fixation: The Occipital Bar-plate and the Occipital Plate. The first one is a moldable bar-plate, which is fixed to the polyaxial screws at its lower end; and to the occipital bone with unicortical screws on its upper end.
The second element is a T-shaped plate with mobile heads. It is fixed to the middle line of the occipital bone. It is connected to the rest of the polyaxial screws with the use of a system-proper bar.
The system also has laminar hooks, transverse connecting hooks, and 3.5mm-diameter longitudinal bars, all manufactured with a titanium alloy (Ti6Al4V ELI, ASTM F-136-02).
The bars can be deformed to adapt to the shape needed for corrections and to maintain appropriate anatomical spine alignments such as lordosis and kyphosis.
For levels C3-C7, Standard Polyaxial Screw design allows for an angulation of 61°±3°. Extreme Polyaxial Screws allow an angulation of 73°±3°.
INDICATIONS
– Malformations
– Post-traumatic instability
– Tumors
– Degenerative conditions
– Anterior fusions needing additional posterior stabilization
TESTS AND STUDIES
– Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model- ASTM F 1717-04.
– ASTM E8 Mechanical and metallurgic characterization
CERTIFICATES
– INVIMA Sanitary Registration Nr. 2018DM-0000666-R1
– ISO 9001 and ISO 13485 Registrations
PRESENTATION AND PACKAGING
Implants from the INFINITY System are provided unsterilized, individually packaged, and individually laser-marked. Each laser marking shows: product code, lot number, MEDIIMPLANTES logo, material symbol (Ti), and use-specific dimensions. Laser markings are permanent and allow for product traceability, even after implantation.
Primary Packaging: medical-grade paper bag for products to be sterilized and a laminated polyester film with a security band. It’s provided with non-toxic chemical indicators, fit for commercial sterilization methods, allowing for internal monitoring of sterilization parameters.
Secondary Packaging: Organizing racks that protect the implant from any possible mechanical damage caused by product movement to other locations.
Tertiary Packaging: Aluminum cases with safety mechanisms that contain all available organizing racks to display in an organized fashion for the surgeon’s use.
STORAGE AND AVAILABILITY
Immediate availability and easy storage. Does not need refrigeration nor any special handling procedures asides from maintaining cleanliness conditions to guarantee sterilization.