System
APOLO
PRODUCT NAME: TRANSPEDICULAR FIXATION SYSTEM
COMMERCIAL NAME: APOLO SYSTEM
REFERENCE: 101
MANUFACTURER: Mediimplantes S.A.
MANUFACTURING MATERIAL: Ti 6Al 4V ELI (Extra low interstitial) Alloy for Surgical Implant Applications – UNS R56401, ASTM F136-02.
FUNCTIONAL DESCRIPTION:
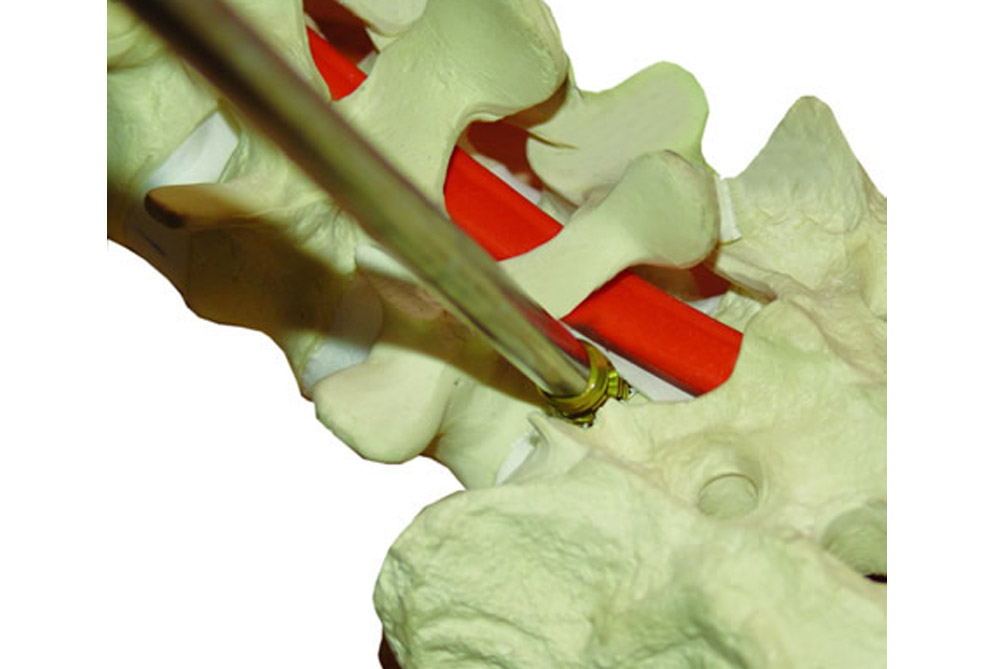
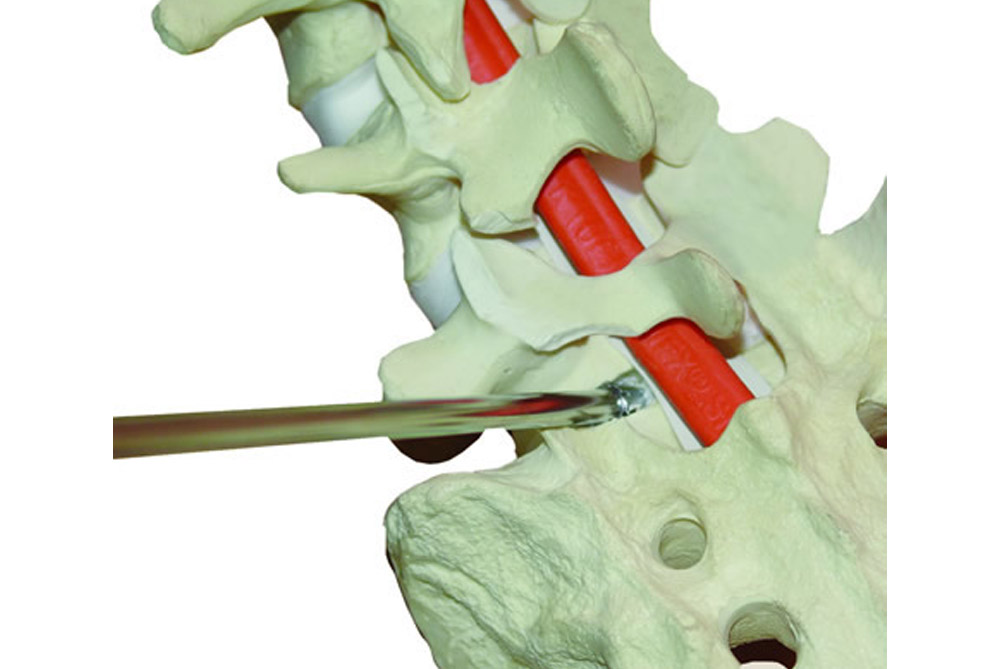
APOLO is a system composed of intervertebral bodies with diameters of 11, 12 and 13 mm., as well as lengths of 20 and 25 mm.
APOLO has a special design that allows to save valuable time by skipping certain steps during surgical procedures. It has a threaded conical end, which allows for slight perforation. Its thread provides good strength and grip.
INDICATIONS:
– Patients that show chronic or severe lower back pain caused by disk degenerative disease.
– Patients with micro-instability.
STUDIES AND TESTS:
– Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model – ASTM F1717- 04.
– Tests Methods for Intervertebral Body Fusion Devices – ASTM F2077.
– ASTM E8 Mechanical and Metallurgical Characterization
CERTIFICATES:
– INVIMA SANITARY REGISTRATION 2007DM-0000663
– ISO 9001 and ISO 13485 Certificates.
PRESENTATION AND PACKAGING
Implants from the APOLO System are provided unsterilized, individually packaged, and individually laser-marked. Each laser marking shows: product code, lot number, MEDIIMPLANTES logo, material symbol (Ti), and use-specific dimensions. Laser markings are permanent and allow for product traceability, even after implantation.
Primary Packaging: medical-grade paper bag for products to be sterilized and a laminated polyester film with a security band. It’s provided with non-toxic chemical indicators, fit for commercial sterilization methods, allowing for internal monitoring of sterilization parameters.
Secondary Packaging: Organizing racks that protect the implant from any possible mechanical damage caused by product movement to other locations.
Tertiary Packaging: Aluminum cases with safety mechanisms that contain all available organizing racks to arrange implant references in an organized fashion.
STORAGE AND AVAILABILITY:
Immediate availability and easily stored. Does not need refrigeration or any special handling besides maintaining cleanliness condition to guarantee sterilization results.